What is phosphorus and what does it do?
What is phosphorus and what does it do?
Phosphorus is a major mineral that is required by all of the cells in the body in order for them to perform their regular functions. The mineral is present in the body as inorganic phosphates in a substantial quantity of about 500-800 g (about 1-2 pounds) in adults, with about 85% of that is found in the bones and teeth with another 10% is found in the muscles or combined with carbohydrates, lipids, and proteins throughout other tissues in the body. The remaining 5% is found in extracellular and intracellular fluids. As an electrolyte mineral, phosphorus is specifically needed to produce energy in cells.
The presence of phosphorus / inorganic phosphate in various forms as a part of cofactors, enzymes, tissues, and bones is practically ubiquitous in the human body. This discussion provides just a brief overview of some of the specific ways in which phosphorus is utilized in the body. For the following discussion, a rudimentary understanding of how phosphorus works is important for clarifying other points I’ll make about this nutrient in the rest of this document. So, below is a list of some of the various other roles that phosphorus plays in the body:
- Phosphorus is a part of cellular membranes in the form of phospholipids like lecithin (phosphatidylcholine). As a nutrient component in phosphatidylcholine, it’s an active participant in facilitating the flow of nutrients and other substances into and out of cells throughout the body.
- Phosphorus found in the phosphate molecule forms the backbone of the nucleic acids DNA and RNA and is therefore essential for cellular reproduction and maintenance.
- Bone/teeth mineralization and remineralization (which is called bone remodeling) is regulated partly by the presence of healthy phosphates in the body.
- Regulation of the pH balance in the body (to ensure that the body doesn’t become either too acidic or too alkaline) is also governed in part by phosphorus.
- In bones and teeth phosphorus is in the form of a calcium phosphate salt.Phosphorus / phosphate and calcium maintain the structural integrity of hydroxyapatite in bones and teeth.
- Phosphorus is a part of adenosine diphosphate (ADP) and adenosine triphosphate (ATP), the primary energy source for all cells in the body. Through the process of phosphorylation (the addition of phosphate to an organic compound), phosphorus plays a role in both the production and storage of energy throughout the body.
- 2,3-diphosphoglycerate (2,3-DPG), a phosphorus-containing molecule that binds with hemoglobin in red blood cells, is partly responsible for the delivery of oxygen to all the tissue of the body.

Click here to schedule a health coaching session with us.
The Differences Between Phosphorus, Phosphate, and Phosphoric Acid
The various terms used to describe phosphorus and phosphorus-containing molecules are thrown around a lot with very little explanation within the context of discussing organophosphates, but below we include a brief description of each of the major terms here:- Phosphorus - This is the inorganic mineral element. It is sometimes known as “inorganic” phosphorus. It is not combined with anything else and could be views as the “pure” form of this mineral. Phosphorus isn’t actually found in the human body in its pure form because it naturally combines with other elements to form inorganic phosphates.
- Phosphate - Phosphate is a form of phosphorus that exists in all living creatures. It combines phosphorus with 4 oxygen atoms. This is the bioactiveform of phosphorus found in the human body, and it may be combined with various other substances to form more complex compounds and molecules. Phosphates are what are used in the Kreb’s cycle to produce cellular energy.Note that there are phosphates that exist naturally (and safely) in the body, and then there are also phosphates that are synthetic and unhealthy for body. There are phosphates that are formed through interactions with other unhealthy substances (for example, the heavy metal aluminum in aluminum phosphate). Any phosphates that are added to food (that don’t already exist naturally in the food, that is) should generally be considered unsafe because they can cause damage to the body over time. Phosphates that exist naturally in food (for example, the phosphates that exist in organic, non-GMO wheat in the form of phytic acid, which can be released and made accessible by sprouting the wheat) are generally harmless and in fact necessary for health.
- Phosphoric acid - Phosphoric acid and the word “phosphate” are technically synonymous, but often, the term phosphoric acid is used to denote orthophosphoric acid. Orthophosphoric acid makes up the esters that form organophosphates, and can be used in fertilizers, insecticides, or food additives. It is toxic to human health.
Phosphorus Deficiency (Hypophosphatemia) Overview
Phosphorus deficiency is relatively rare, since phosphorus exists in one form or another in nearly every food that we eat. This is why deficiency in phosphorus is unusual. However, it is still technically possible to have a phosphorus-deficiency, though it only generally happens in extreme circumstances (some of which I’ve listed below).When a person has a phosphorus deficiency, this can lead to a depletion in ATP, and therefore a decrease in cellular energy (which causes many of the symptoms listed below, including those related to organ failure, since the organs of the body require ATP in order to carry out their jobs). Deterioration of bones and teeth can also occur because if phosphorus isn’t present, calcium cannot bind with it to form the strong crystals that make up strong bones and teeth.
Phosphorus Deficiency Symptoms
Hypophosphatemia symptoms (phosphorus deficiency symptoms) include those such as:- Loss of appetite (anorexia)
- Autoimmune disease
- Low immunity
- Respiratory failure
- Rickets (in children) / vitamin D deficiency
- Note that there is a relationship between phosphorus / phosphate and vitamin D levels in the body. Also note that vitamin D is a nutrient that is produced naturally in the human body when the skin is exposed to sunlight (without sunscreen).
- Bone disease
- Bone pain
- Osteomalacia
- Osteoporosis
- Fragile bones
- Other bone diseases
- Bone or blood cancers
- Leukemia
- Lymphoma
- Multiple Myeloma
- More…
- Blood disease
- Anemia
- Hemolysis
- More…
- Nervous system disease
- Confusion
- Central pontine myelinolysis
- Seizures / epilepsy symptoms
- Peripheral neuropathy / nerve pain
- Guillain-Barre symptoms
- More…
- Diseases that involve muscle problems
- Difficulty walking / muscle coordination issues
- Muscle weakness
- Numbness and/or tingling in the extremities
- Rhabdomyolysis
- Cardiovascular disease
- Cardiac muscle failure and heart failure
- Heart arrhythmia

Click here to learn more about the DreamLight.app, a guided meditation and brain-entrainment tool that enlists the mind and spirit in the healing process to overcome cancer naturally.
Phosphorus Deficiency High Risk Groups
Below are some specific risk groups that may have a higher likelihood of developing phosphorus deficiency or suboptimal phosphorus levels. While not everyone who falls into these categories will develop a formal phosphorus deficiency, the likelihood of depleted phosphorus levels is higher if a person falls into one of these groups.These risk groups include:
- Alcoholics
- Diabetics (specifically those who have recently experienced diabetic ketoacidosis)
- Patients with respiratory alkalosis
- Individuals experiencing starvation
- Anorexics
- People with phosphorus wasting disorders
- Postmenopausal women receiving hormone replacement therapy (these women may have higher levels of urinary phosphorus excretion and therefore lower serum phosphorus levels)
- Chronic use of aluminum-containing antacids or calcium carbonate antacids (aluminum binds with phosphorus to form aluminum phosphate, a form of phosphorus that is not able to be absorbed in the body, meaning that less phosphorus in food can be absorbed and that phosphorus levels may become suboptimal over time; calcium carbonate also binds with phosphorus, potentially resulting in similar issues).
 Click here to subscribe to the Living Database!
Click here to subscribe to the Living Database!
Phosphorus Toxicity (Hyperphosphatemia) Overview
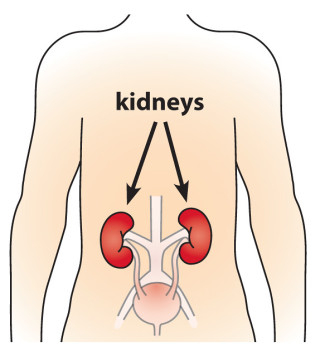
Phosphorus toxicity is also known as hyperphosphatemia. This condition is considered by conventional medicine to be uncommon since, in healthy people, the kidneys are supposed to excrete excess phosphorus via the urine, ensuring that phosphorus levels are maintained at an appropriate level. In individuals with impaired kidney function, however, hyperphosphatemia is significantly more likely and it may cause symptoms like those below (note that the symptoms with an “ * ” and manifestations of resulting hypocalcemia caused by high phosphorus levels):- Calcium deposits / Ectopic calcification
- Hardening (calcification) of soft tissues, specifically in the kidneys
- Cardiovascular disease
- Secondary hyperparathyroidism
- Renal osteodystrophy / Adynamic bone disease
- Seizures*
- Coma*
- Diarrhea
- Interference with the body’s ability to use iron, magnesium, and zinc
- Muscle atrophy
- Skin atrophy
- Premature aging
- Coronary calcifications in young, otherwise healthy men
- Endothelial dysfunction
- Heart attack
- Bone-related problems
Causes of phosphorus toxicity / hyperphosphatemia may include:
- Kidney failure
- Rhabdomyolysis
- Diabetic ketoacidosis
- Tumor lysis syndrome
- Hypoparathyroidism
- Pseudohypoparathyroidism
- Excessively high levels of vitamin D
- Use of some pharmaceutical drugs, such as penicillin, corticosteroids, some diuretics, furosemide (Frusol, Diuresol), and thiazides (Diuril, Aprinox, etc).
Though conventional medicine claims that phosphorus toxicity is rare, I don’t personally believe that this is necessarily the case. After all, the organophosphates and phosphate food additives are absorbed in significantly higher quantities than the natural phosphates that are found in natural, whole foods. This may be due, in part to the widespread problem of vitamin K2 deficiency and Lugol’s iodine deficiency, two nutrients that help the body balance calcium and phosphorus levels. But if you’re following a model of a person with optimal health who eats only fresh, organic produce and grass-fed, antibiotic free animal products, and who has never taken a pharmaceutical in their life and who lives in an environment that’s free of contamination with any organophosphates (the Hunza tribe in India/Nepal are the only example I can think of where this might be the case), then I would agree with conventional medicine’s assessment. Yes, hyperphosphatemia in this person’s situation would be pretty unusual.
But most of us don’t have the luxury of being able to live lives that are free of contamination. Even if a person takes care to eat only the freshest and best foods and to stay away from organophosphate-containing pharmaceuticals and other chemical sources of phosphate, they’re still likely to be exposed to organophosphates in one way or another. These substances are prevalent in most developed countries, as well as in some developing countries. And again, because they’re more easily absorbed by the human body than other natural phosphates, particularly in individuals who are deficient in vitamin K2 and Lugol’s iodine, their presence ultimately, in some ways, increases the risk of phosphorus toxicity for everyone, regardless of lifestyle or location.

The AlivenHealthy Iodine Bible - Everything you need to know to get started taking iodine and more!
What is the difference between natural phosphates and synthetic phosphates (organophosphates, bisphosphonates, etc)?
Natural phosphates (which are sometimes referred to as inorganic phosphates) are found in the body, bound to molecules like ATP, phospholipids, and DNA, where they perform a wide variety of tasks. When separate from other molecules, natural phosphate is made up of a phosphorus atom and 4 oxygen atoms. These natural, inorganic phosphates are also present in most foods in some form, since all living beings and cells need phosphorus to survive, where they are also then found bound into different compounds that can be absorbed and then used by the human body in the appropriate quantities.Organophosphates, in contrast, are made up of a phosphate molecule (1 phosphorus and 4 oxygen atoms) bound to a hydrocarbon. Because these molecules contain carbon, they’re referred to as “organic”. Natural, inorganic phosphates are found in nature and are beneficial for human health though, while organophosphates are synthetically produced and are used as chemical fertilizers and insecticides, flame retardants, and nerve gases (among other things).
Natural phosphates support life. Organophosphates destroy it.
Bisphosphonates (also known as diphosphonates) are pharmaceutical drugs used to treat conditions such as osteoporosis, primary hyperparathyroidism, fibrous dysplasia, Paget’s disease of the bone, and more. They are made up of two phosphonate groups, which are organophosphorus compounds. Bisphosphonates are intended to be quickly absorbed into the bone tissues. Once they’re absorbed, without other detoxifying treatments, it can take 10 years or more for them to be eliminated from the body. We discuss bisphosphonates in more depth here.
Phosphate Food Additives vs. Natural Phosphates in Food
Phosphates food additives are extremely common. Most of these unnatural phosphates are used to make foods creamy, to extend their shelf life, to keep powdered foods from clumping, or to enhance the food’s flavor or texture. While these kinds of phosphates are most commonly found in packaged and/or processed foods, they also are sometimes found in common packaged “healthy” food products that appear to be whole and are sold unpackaged and unchanged from their natural state, including meats, poultry, and seafood, where organophosphates injected into the meats to retain moisture (note that producers are not required to list phosphates used in this way as an ingredient in the meat).Phosphate food additives are found in many different foods, and can include ingredients like:
- Sodium phosphate (E339)
- Potassium phosphate (E340)
- Calcium phosphate (E341)
- Orthophosphoric acid diphosphate (E450), triphosphate (E451), and polyphosphate (E452)
- Tricalcium phosphate (E341)
- Phosphoric acid (E338)
- Ferric phosphate
- Sodium tripolyphosphate (E451)
- Dipotassium phosphate (E340)
- Pyrophosphate (E450)
- Hexametaphosphate (E452)
- Tetrasodium phosphate (E450)
- Sodium acid pyrophosphate (E450)
Natural phosphates in natural foods are very different from the phosphate-based food additives found in processed foods like deli meats, sodas, packaged desserts, and others. One important fact is that natural phosphates are only partially absorbed in the intestines. Naturally-occurring phosphates in meats and other protein-rich foods may be 40-60% absorbed, while those found in fruits and vegetables are only about 50% absorbed. In grains and legumes, the main source of phosphorus is found in phytic acid, a compound that isn’t usually absorbed at all unless the grains/legumes have been soaked or sprouted to release the phosphorus present. This diminished phosphorus bioavailability in most natural foods acts as a defense/balancing mechanism that our bodies have developed in harmony with the Plant Kingdom over thousands of years to ensure that we get plenty of this necessary mineral in our diet without accidentally ingesting too much.
In contrast with naturally-occurring phosphorus found in fruits and veggies or animal products, phosphate-based food additives like those listed above are much more effectively absorbed/bioavailable (in fact, they can be absorbed at a rate of up to 90% in the intestines!). This is because phosphate food additives aren’t bound up into the same type of molecules that are in natural foods like fruits and vegetables (i.e. phytic acid), so they’re ultimately much easier for the body to absorb. And, since manufacturers aren’t required to label the amount of additional phosphorus that’s added to packaged, processed foods, consumption of these phosphates can add up fast, leading to higher-than-normal phosphorus levels in the body (and the hyperphosphatemia symptoms that accompany the problem).
Food manufacturers aren’t required to list the amount of phosphorus in a given food product in the Nutrition Facts section. Some manufacturers do, but most don’t. While you may be able to see that there are phosphates in a food in the ingredients section, there’s not any easy way to determine the actual amount of phosphates in the food, meaning that it’s easy to consume far more than is healthy. The safe upper limit of phosphorus intake is 4000 mg/day, and the Acceptable Daily Intake (ADI) for phosphorus is currently set at 40 mg/kg of bodyweight (which averages out to 2800 mg per day for a 70kg adult). Experts agree that our phosphorus intake can be lower than this though, and that around 700 mg daily is a good amount to aim for. For reference, it’s not uncommon for the average American to consume around 1000 mg of phosphates per day, and this doesn’t even include the phosphorus that we get from food additives.
Keep in mind that even if you place a high value on eating healthfully and avoiding processed foods, chances are good that you have phosphate food additives in the food in your pantry right now and have never paid them any mind. Most people know or have a vague awareness that phosphorus is necessary for health, and because most of us are also aware that nutrients are sometimes added to foods to fortify the we overlook phosphate food additives because, on some level, we automatically assume that they’re just another nutrient fortification that’s “good for us”.
Do you drink vegan milk from time to time (or daily)? Check out the ingredients. Silk, a popular vegan milk brand, includes tricalcium phosphate in its “Vitamin and Mineral Blend” for its Original Soymilk product. That’s tricky, right? If a company lumps together an ingredient like tricalcium phosphate with others like vitamin B12 and vitamin B2, then most consumers will automatically think that the phosphate additive is nutritive and therefore healthy. Chances are, it isn’t nutritive at all, since most people eating a healthy, varied diet get more than enough phosphorus. The tricalcium phosphate, in this context, is likely added to the milk as a stabilizer or to ensure that the milk remains creamy and doesn’t separate.
This particular vegan milk brand’s Coconutmilk and Oatmilk products contain dipotassium phosphate (this time not a part of the Vitamin and Mineral Blend), also likely added to enhance creaminess, prevent separation, or lengthen shelf-life. Other vegan milk brands also contain these ingredients (of course, not all vegan milks contain phosphates, but their ingredients deserve a second look if you have vegan milk in your pantry). Other so-called healthy products that come in a package (don’t be fooled, that’s still a package around your store-bought nut milk, marketing is a tricky, tricky thing) also are highly likely to contain at least one phosphate food additive ingredient.
I want to delve further into this problem, though. Because the issue doesn’t stop with phosphate food additives. While added phosphates pose their own set of issues, which I’ll discuss in more detail below, there are more layers to the problem here. Let’s go back to the example of Silk’s Original Soymilk and actually look at the ingredients:
- Soymilk (Filtered Water, Soybeans)
- Cane Sugar
- Vitamin and Mineral Blend
- Tricalcium Phosphate
- Calcium Carbonate
- Vitamin A Palmitate
- Vitamin D2
- Riboflavin [B2]
- Vitamin B12
- Sea Salt
- Natural Flavor
- Gellan Gum.
Not so bad, right?
Well, you’d be partially right. In comparison to other packaged foods, this particular product is relatively healthy. If you drank a glass of it maybe once a month, there’d be no real issue. However, if you were to drink this milk daily in larger amounts, there are some important problems with it that could potentially cause health problems under certain circumstances.
To start, there’s nothing wrong with filtered water and soybeans, or with gellan gum, or with sea salt. Riboflavin (vitamin B2) and vitamin B12 are also acceptable additions. Vitamin A palmitate is still a natural form of vitamin A, however this is an active form of the vitamin, meaning that it is significantly more bioavailable than the inactive form, beta-carotene. In general, I prefer to supplement with beta-carotene since it’s safer, but in the context of food fortifications, I can see the reasoning in including the active, available form of vitamin A. I don’t like the addition of sugar, but since people who are reading this probably know something about the negative effects of refined sugar on health, I’m not going to digress into talking about refined sugars in this discussion.
Natural flavor is a deceptive term. It can encompass a wide variety of ingredients, including some natural, harmless ingredients such as spices, as well as ingredients that some people may prefer not to consume, such as monosodium glutamate (MSG) or its byproducts, diacetyl (a “natural” butter flavoring), and others. Some sources have even said that, in reality, natural flavorings aren’t all that different from artificial flavorings.
That leaves tricalcium phosphate, calcium carbonate, and vitamin D2. As we’ve already discussed earlier in another discussion, the combination of calcium and vitamin D without vitamin K2 can cause serious health issues, including diseases like atherosclerosis, osteoporosis, dementia, and others. Read more about the dangers of supplementing with vitamin D without the balancing effects of vitamin K2 here. Add a highly bioavailable form of phosphate into the mix, and you’ve got a real problem, since excess phosphorus can exacerbate signs and symptoms of hypercalcemia (high calcium levels/calcium toxicity). So, the calcium and vitamin D2 alone without vitamin K2 is negative, but the addition of phosphorus ultimately makes the situation much worse, as we’ll soon see. In later parts of this article, we’ll talk more about the intricate interactions between these three nutrients.
The Connection Between Phosphorus and Vitamin D
NOTE: What follows is not an endorsement for pure vitamin D supplementation… vitamin D should only be supplemented in combination with vitamin K2. Taking a combined vitamin K2+D3 supplement is acceptable (and good, in fact), but do not take a vitamin D supplement on its own without vitamin K2 since this can cause serious health problems, including atherosclerosis, porcelain gallbladder, calcium deposits under the skin (like those seen in dermatomyositis), etc. Read more about the use of vitamin K2 to cure leukemia here.Learning about the interactions between different nutrients in the body can be complex and hard to follow, but these interactions are extremely important for being able to understand how a single nutrient works in the body to maintain (or, in some cases, to destroy) health. So, I’ve included this section to try and illuminate some of the ways that phosphorus interacts with other nutrients in the body (and therefore, how phosphorus itself works). Just like how it’s easier to understand a person when you consider the other people they interact with in their daily life, it’s easier to understand a nutrient when you consider the other nutrients that exist like a community around phosphorus.
Phosphorus, calcium, and vitamin D are closely related to each other. Vitamin D deficiency can start a chain reaction in the body where blood phosphorus levels increase above average, and subsequently, blood calcium levels decrease beyond where they should. This is because active vitamin D helps regulate phosphorus levels. Without sufficient vitamin D, phosphorus levels can increase beyond the normal range. Phosphorus, in turn, supports the excretion of calcium from the body. When these three nutrients are balanced, they can keep each other in check (the vitamin D makes sure that phosphorus doesn’t get out of control, and the phosphorus is then kept at a level that ensures that the appropriate amount of calcium is removed from the body). But, when vitamin D levels get too low, this can ultimately lead to a calcium deficiency since the higher-than-normal phosphorus levels ultimately lead to more calcium excretion than normal.
Parathyroid hormone (PTH) and boron (a trace mineral that regulates parathyroid health in a similar way that iodine regulates thyroid health) also play important roles in the balance of these three nutrients. When calcium levels drop slightly below where they should be, the parathyroid senses this and releases PTH in response. The increased levels of PTH cause the body to excrete less calcium via the urine, and to increase the excretion of phosphorus. PTH in this case also stimulates the reabsorption of minerals into the bones (so if calcium has been lost from bones, it can thus be replaced). In addition, PTH activates vitamin D, thereby opening another line through which phosphorus balance can be maintained. We talk in depth about the balance between phosphate, calcium, boron, and other relevant nutrients here.
Boron, an essential trace mineral, is then important not only because it helps maintain parathyroid hormone health, but also because it interacts directly with the other nutrients we’re discussing here. When boron is taken as a supplement, studies have demonstrated that it decreases the rate at which calcium and magnesium are lost in the urine (meaning these minerals will be present in higher quantities in the body), and also that it generally decreases phosphorus levels in the urine. Boron is also essential in order of the body to make use of both vitamin D and calcium.
Bringing this discussion back to phosphorus toxicity though (which is where this information is especially relevant)… based on the nutrient interactions above, there’s more than one way that a person may develop some kind of an imbalance in phosphorus levels. For example, individuals with autoimmune conditions tend to have problems absorbing (and in this situation, producing/activating) fat-soluble vitamins like vitamin D. Though these people may be able to make vitamin D in the skin through sunlight exposure, they may not be able to absorb vitamin D in nutrient form. Therefore, it’s possible that these people may develop problems related to excess phosphorus in the body and/or depleted calcium levels. People with hyperparathyroidism or hypoparathyroidism may also have issues with phosphorus balance, since, as I outline above, parathyroid hormone is necessary for the balance of active vitamin D, calcium, and phosphorus.
The Connection Between Organophosphates, Acetylcholine, and Calcium
Organophosphates are well-known for their cholinesterase inhibitory action. Cholinesterase is an enzyme that facilitates the breakdown of acetylcholine; without it, the neurotransmitter acetylcholine would build up in the brain and nervous system, leading to symptoms of cholinergic toxicity. Some of the symptoms of cholinergic toxicity include (but are not limited to):- Involuntary twitches or movements
- Anxiety
- Confusion
- Dizziness
- Difficulty concentrating
- Irritability
- Circulatory and respiratory depression
- Bradycardia
- Hypertension
- Restlessness
- Insomnia
- Seizures
- Coma
- Areflexia / Lack of reflexses
- Bronchospasm
- Blurred vision
- Urinary incontinence
- Vomiting
- Diarrhea
- Muscle rigidity
Chronic, long-term exposure to organophosphates may result in some of the milder symptoms of cholinergic toxicity that are practically unnoticeable, or a person may end up in a doctor’s office and be diagnosed with a specific disease such as myasthenia gravis (or any number of diseases) though the actual problem involves chronic exposure to toxins, not autoimmunity. Organophosphates are, of course, harmful to human health (in more ways than one). But, while we absolutely need acetylcholine, too much can be detrimental, so cholinesterase is there to keep things in check. Organophosphate exposure disrupts this system of checks-and-balances by inhibiting the production and activity of cholinesterase.
There are 2 types of acetylcholine receptors: nicotinic and muscarinic. These receptor types are located in different areas of the body; muscarinic receptors, for example, are located in the glands, neurons, cardiac muscles, and smooth muscle tissues, and nicotinic receptors are found in the skeletal muscle and other areas of the nervous system. They also serve different functions. The nicotinic receptors are responsible for rapid neurotransmitter transmission and the muscarinic receptors, in contrast, are responsible for managing a slow metabolic response.
When acetylcholine overstimulates nicotinic receptors in particular, this stimulates the build-up of calcium inside of cells. While normally there’s a delicate cycle that encourages the gradual build-up and subsequent release of calcium inside of cells, cholinergic toxicity disrupts this cycle. In fact, excess acetylcholine halts the cycle entirely, which is what causes the calcium build-up in intracellular fluids.
Calcium build-up in the cells, when left unattended for a long period of time, can cause calcifications of the cellular organs, such as the mitochondria. Mitochondria is like the battery of the cell so calcification of this structure can eventually kill the cell. Not only do these calcifications eventually cause cell death, but they can also eventually lead to calcifications of tissues and even entire organs which cause symptoms of a wide range of diseases and disorders. Read more about the consequences of pineal gland calcification here.
Organophosphate exposure isn’t the only reason why our bodies develop a heavy phosphate load or hyperphosphatemia, but its important to understand the idea that food additives and other sources of phosphates that are unnatural are still an important piece of the puzzle when it comes to why organophosphates are so bad. Phosphorus toxicity from phosphate food additives or, say, a phosphorus supplement (NOTE: don’t take phosphorus as a supplement) ultimately can cause an increase of calcium in the blood through a complex set of pathways involving a depletion of magnesium and other minerals. When you consider this fact, and then the idea that organophosphates not only contribute to phosphorus toxicity, but also stimulate the entry of calcium into cells, it’s easier to see how organophosphate exposure over the long-term (in combination with other types of excess phosphate exposure) could cause serious health issues.
Root Cause: Common Environmental Toxins and How to Protect Yourself From Them - BUY HERE!
Related Posts:Resources: