How to Find the Right Dose: Chlorine Dioxide / Miracle Mineral Solution
How to Find the Right Dose: Chlorine Dioxide / Miracle Mineral Solution
Dr. Thomas Hesselink provides one of the most comprehensive web sites on the topic of Chlorine Dioxide for medicinal use along with other oxidant medicines. He offers a Chlorine Dioxide protocol with precise dosing for patients based on their body weight. His protocol is based on Jim Humble’s findings, but Dr. Hesselink takes his findings to a much more technical and detailed level. His web site describes oxidant biochemistry in great detail.
Dr. Hesselink’s protocol was designed specifically for physicians who wish to administer Chlorine Dioxide Solution or Sodium Chlorite Solution to patients.
What is Sodium Chlorite and how is it related to Chlorine Dioxide?
Sodium chlorite is one of two ingredients that must be combined to produce chlorine dioxide. Typically, when a person buys “chlorine dioxide” online they are actually purchasing a water purification system that will arrive at their doorstep as two ingredients: sodium chlorite and an acid (e.g. hydrochloric acid, citric acid, vinegar, etc.). The sodium chlorite and the acid must be combined in a particular way to produce chlorine dioxide.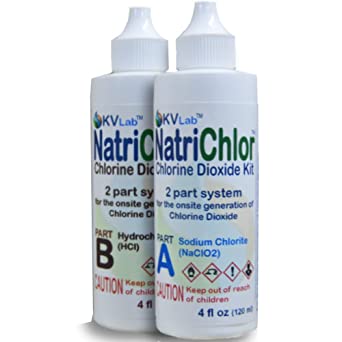
Buy CDS / MMS here. Our Amazon CDS / MMS links to product often disappear shortly after we publish. Contact us at [email protected] to get a link to CDS / MMS for human use.
Jim Humble and Dr. Andreas Kalcker describe different methods for combining sodium chlorite with an acid to activate these two ingredients in order to produce chlorine dioxide. Dr. Hesselink mostly follows the system outlined by Jim Humble, but he uses tools or precision to measure the sodium chlorite and the acid which we describe below. These tools and his algorithms are useful for patients who wish to administer chlorine dioxide in precise doses that are exactly the same as what Jim Humble recommends for different diseases.Sodium chlorite, for all intents and purposes, is the most important ingredient in the Chlorine Dioxide solution. The acid agent (citric acid, vinegar, etc.) is used to “activate” or “potentiate” the sodium chlorite and to make it stronger. Dr. Thomas Hesselink explains that acids cause oxides of chlorine to become more reactive. Bases (which are alkaline), in contrast, cause oxides of chlorine to become more stable.
Below, we detail a process through which you can determine a precise dose of sodium chlorite for yourself or a loved one and then add the acid activator just prior to administering it.
How to Prepare Sodium Chlorite Solutions for Hesselink’s Protocol:
Dr. Hesselink describes a low-cost procedure for preparing chlorine dioxide for oral ingestion. He suggests that patients seek out “technical grade” sodium chlorite. Technical grade sodium chlorite in powder or flake form. This sodium chlorite powder consists of the following components:- Sodium chlorite (NaClO2) - 80%
- Sodium chloride (NaCl) / Table Salt - about 19%
- Sodium hydroxide (NaOH) - less than 1% - the hydroxide stabilizes the chlorite (ClO2) that forms in water.
- Sodium chlorate (NaClO3) - less than 1%
- Chlorate (ClO3) - a harmless excipient that is leftover from the manufacturing process.
If you prefer to buy the sodium chlorite solution pre-made, that’s okay. Many people feel more confident about using Chlorine Dioxide solution when they buy the two-bottle set that’s made for water purification. The two-bottle liquid set works fine for many people and Dr. Hesselink’s precision dosing protocol can still be used by people who choose to use the two bottles of liquids. If you go this route, skip the following steps for making the sodium chlorite solution and go to the section on calculating sodium chlorite dosage based on body weight.
Step 1: How to Make Sodium Chlorite Solution from Sodium Chlorite Powder
Humble’s chlorine dioxide formula is made from 28% technical grade sodium chlorite. This formula consists of 22.4% sodium chlorite which can be derived by multiplying 28% times 80% (because only 80% of the flakes/powder is sodium chlorite, the remaining 20% are relatively inactive ingredients). As such, every milliliter of the sodium chlorite solution contains 224 mg of sodium chlorite.Dr. Hesselink discusses the problem of dropper bottles and the differences between them in terms of the size of drops that are dispensed from the different chlorine dioxide bottles on the market. Jim Humble preferred a dropper that dispensed 25 drops for every cc/mL. Accordingly, if you were using equipment that is identical to Humble’s, each drop of your Chlorine Dioxide would contain 9 mg of sodium chlorite per drop (224 mg / 25).
Most people do not use equipment that is similar, let alone identical to Jim Humble’s, but Dr. Hesselink suggests that individuals can obtain graduated pipettes and syringes to measure the sodium chlorite solution precisely to get very close to Jim Humble’s exact protocols for curing disease. A drop from one dropper is not the same size or the same dose as a drop from a dropper provided (for example) by a given Chlorine Dioxide brand. Droppers vary in terms of the amount of sodium chlorite that they would produce per measure of volume (usually between 15 and 30 mg per mL). But you don’t have to use the dropper provided in a bottle of pre-made sodium chlorite liquid. You can use a syringe rather than a graduated cylinder to administer precise doses according to the instructions below. Please note that you will NOT inject the chlorine dioxide or even use the needle. You will merely use the syringe as a convenient way to measure your sodium chlorite solution to determine how big the drops are that are produced by the specific dropper that you plan to use when dosing your chlorine dioxide. Basically, the syringe functions like a graduated cylinder, but syringes are much easier to obtain because they’re available at most pharmacies throughout the world.
- Get either a milliliter measuring cup or a syringe that provides cc/mL measurements
- Take the plunger out of the back of the syringe.
- Add water from the dropper you will be using to dose your Chlorine Dioxide to the syringe via the top of the syringe (as opposed to sucking the water up through the needle). Remember, this step in the process is merely to measure the actual volume of the drops produced by the dropper you’ll use to dose your chlorine dioxide later in this process–you will only need to do this part of the process one time.
- Count how many drops it takes to reach the 1 cc/mL line on the syringe.
- Divide the number of drops into the known number of milligrams per cc/mL of solution. This will give you the exact milligrams per drop of sodium chlorite.
Step 2: Hesselink’s Recipe and Dosing Strategy for Sodium Chlorite Solution Preparation (Before Activation with the Acid)
Use a gram scale to weigh 25 grams of technical grade sodium chlorite. Dissolve the 25 grams of sodium chlorite in 100 cc/mL of water (you can use your syringe to measure the water precisely). The combination of 25 grams of sodium chlorite mixed into 100 cc/mLs of water will produce a 25% solution of sodium chlorite. Since technical grade sodium chlorite only contains 80% sodium chlorite along with other ingredients, the actual concentration of sodium chlorite is 25% x 80% = 20%. Thus, this solution contains 200 mg of sodium chlorite per cc/mL.Step 3: Using a Graduated Pipette to Add the Acid Activator and Make Chlorine Dioxide
You can use a graduated pipette to measure a precise amount of sodium chlorite, which is medicinal ingredient that will activate with an acid in the final step.This protocol is a precise method of dosing sodium chlorite for both adults, infants, and children. Hesselink recommends a dose between 20 mg to 200 mg for adults. Smaller doses can be administered as 10% or 5% sodium chlorite solutions.
Small children and infants will require a much lower dose of sodium chlorite to treat disease. Prepare a 5% or 10% solution for young children or infants. For both adults and children, the proper medicinal dose is 1 mg of sodium chlorite per 1 kg of body weight per day. For more information about how to calculate the proper dose of sodium chlorite per kilogram of body weight see the next section.
For a 20% sodium chlorite solution:
0.2 cc/mL = 40 mg sodium chlorite 0.3 cc/mL = 60 mg 0.4 cc/mL = 80 mg 0.5 cc/mL = 100 mg 0.6 cc/mL = 120 mg 0.7 cc/mL = 140 mg 0.8 cc/mL = 160 mg 0.9 cc/mL = 180 mg 1 cc/mL = 200 mg
For a 10% sodium chlorite solution:
0.2 cc/mL = 20 mg sodium chlorite 0.3 cc/mL = 30 mg 0.4 cc/mL = 40 mg 0.5 cc/mL = 50 mg 0.6 cc/mL = 60 mg 0.7 cc/mL =70 mg 0.8 cc/mL = 80 mg 0.9 cc/mL = 90 mg 1 cc/mL = 100 mg sodium chlorite
For a 5% sodium chlorite solution:
0.2 cc/mL = 10 mg sodium chlorite 0.3 cc/mL = 15 mg 0.4 cc/mL = 20 mg 0.5 cc/mL = 25mg 0.6 cc/mL = 30 mg 0.7 cc/mL = 35mg 0.8 cc/mL = 40mg 0.9 cc/mL = 45 mg 1 cc/mL = 50 mg sodium chlorite
Step 4: How to Calculate the Appropriate Dose of Sodium Chlorite Based on Body Weight:
To calculate the appropriate dose of sodium chlorite for you or a loved one, begin by weighing yourself. If you weighed yourself in pounds, divide that number by 2.2 to determine your weight in kilograms. In this step, you will still be working with just the sodium chlorite solution. After you have found the appropriate dose of sodium chlorite (which is measured in milligrams per kilogram of body weight), then, in the next step, you’ll add an equal amount of activator solution (citric acid, vinegar, hydrochloric acid, etc.) to produce the Chlorine Dioxide just a few minutes before taking the dose.EXAMPLE:
An adult who weighs 187 pounds would divide 187 pounds by 2.2 (the formula for changing pounds into kilograms). 187 / 2.2 = 85 kilograms. In other words, an adult who weighs 187 pounds also weighs 85 kilograms.
The formula for changing pounds into kilograms is:
weight of the patient in pounds / 2.2 = weight of the patient in kilograms
According to Dr. Hesselink, an appropriate sodium chlorite dose is approximately 1 mg sodium chlorite per kg of body weight per day. This is true for both adults and children as well as infants. This is the optimal dose to produce a medicinal effect in the body using the sodium chlorite/chlorine dioxide as a starting dose. You will increase the dose over time within a range that will continue to be beneficial medicinally.
As such, an adult who weighs 85 kg should START sodium chlorite treatment at 85 mg per day. Ideally, this treatment would be administered in divided doses throughout the day. This hypothetical adult could work up to a maximum of 170 mg per day over time.
Now let’s consider a 16 lb infant. First, we would need to convert the weight from pounds into kilograms:
16 pounds / 2.2 = 7.27 kilograms
The correct starting dose for this 16 pound infant would therefore be 7.27 mg of the sodium chlorite solution.
Overdose Information for Sodium Chlorite
An overdose of sodium chlorite can be deadly and taking too much (or too little sodium chlorite) is counter-productive in terms of how the immune system responds to the treatment. Taking too much sodium chlorite can cause an overactive immune response (cytokine storm) that would obviously be undesirable. For best results, sodium chlorite should be administered within the optimal dosage range, according to Dr. Hesselink’s.The lethal sodium chlorite dose is approximately 100 mg per 1 kg of body weight. It is difficult to overdose on sodium chlorite, but of course it’s possible. Anyone who is working with an infant or a child, someone who is very weak or debilitated, or elderly individuals can understand the value of being able to properly dose sodium chlorite for each administration of chlorine dioxide.
Ingesting 8 -10 grams of sodium chlorite could kill an average sized adult. This is approximately 100 times the appropriate dose.
On the other hand, a baby could be killed by taking as little as 400 mg of sodium chlorite.
Recommended Daily Sodium Chlorite Dose by Body Weight
Wt. Lbs. Wt. Kgs Start Dose Ave. Dose Max Dose Lethal Dose9 lb. 4 kg. 1 mg 4 mg 8 mg 400 mg
13 lb 6 kg 1.5 mg 6 mg 12 mg 600 mg
22 lb 10 kg 2.5 mg 10 mg 20 mg 1 gram
30 lb 14 kg 3.5 mg 14 mg 28 mg 1.4 grams
44 lb 20 kg 5 mg 20 mg 40 mg 2 grams
66 lb 30 kg 7.5 mg 30 mg 60 mg 3 grams
100 lb 45 kg 11 mg 45 mg 90 mg 4.5 grams
154 lb 70 kg 17.5 mg 70 mg 140 mg 7 grams
220 lb 100 kg 25 mg 100 mg 200 mg 10 grams
Adding the Acid Activator to the Sodium Chlorite Solution
When you add acid to sodium chlorite right before using it, the acid enhances the effectiveness of this medicine. This is why it is called an “activator” when sodium chlorite is being used as a medicine. Adding an acid to sodium chlorite causes most of the sodium chlorite to turn into Chlorine Dioxide. Dr. Hesselink notes that Jim Humble’s favorite Chlorine Dioxide formulation was made from 28% technical grade sodium chlorite in water.Once you’ve determined the proper dose of sodium chlorite (NaClO2) for the patient and measured it to determine the dose supplied by your dropper, you should put this dose into a glass container. In this step of the process, the acid activator will be used to create a pH between 2 and 3 which is acidic. The sodium chlorite will be most reactive with the acid activator at a pH between 2 and 3 which basically means that you’ll produce the most Chlorine Dioxide from the reaction if you add an acid activator that creates this pH when mixed with sodium chlorite. You can use pH paper to check the pH of the solution. The acid activators that can be used to activate sodium chlorite include:
- Lactic acid
- Acetic acid
- Tartaric acid
- Citric acid 5%, 10%, or 20% (Citric Acid 10% is the preferred activator according to Dr. Hesselink)
- Diluted hydrochloric acid (use caution during preparation with stronger acids)
- Diluted sulfuric acid (use caution during preparation with stronger acids)
- Diluted phosphoric acid (use caution during preparation with stronger acids)
- Other strong edible acids that are not toxic.
To prepare a 10% citric acid solution, add 1 part citric acid to 9 parts reverse osmosis or filtered water. Use your gram scale to measure the citric acid. Use a measuring device with mL markings to measure the water according to the following chart:
Citric Acid Total Volume in Millimeters of the Final Citric Acid Solution
Strength
500 mL 1000 mL 2000 mL
5% 25.5 g Citric Acid 51 g Citric Acid 102 g Citric Acid 484 mL Water 969 mL Water 1939 mL Water
10% 52 g Citric Acid 104 g Citric Acid 209 g Citric Acid 468 mL Water 937 mL Water 1875 mL Water
20% 109 g Citric Acid 218 g Citric Acid 436 g Citric Acid 435 mL Water 869 mL Water 1738 mL Water
Achieving an acidic pH between 2 and 3 is vital for optimal production of Chlorine Dioxide from your sodium chlorite solution. The volume of acid activator should be carefully calibrated because too large of a volume of acid will actually diminish the rate with which chlorine dioxide is produced. This is chemistry and the goal here is to be precise to achieve the best results from each administration of Chlorine Dioxide.
Dr. Hesselink recommends the use of 10% citric acid at a volume equal to the sodium chlorite solution as the ideal acid activator. Citric acid at 20% concentration would react more quickly than the 10% concentration of citric acid. He recommends that the sodium chlorite and the acid activator react as a mixture (swirl it around in the glass) for 3 minutes. This final solution smells like elemental chlorine (Cl2).
After the 3 minute reaction time between sodium chlorite and the acid activator, the solution will contain mostly chlorine dioxide along with small amounts of unreacted sodium chlorite, table salt (sodium chloride), sodium citrate, and citric acid. The solution should be a yellow to brown / orange color. It must now be diluted with water. Adding a moderate amount of water to the solution before drinking it helps prevent nausea when taking this medicine. For most adults adding 1 cup of water to the activated Chlorine Dioxide solution is about the right amount to prevent the throat and mouth from becoming irritated. Children will need drink less than 1 cup of water per dose depending on their size. The 1 cup solution tastes like salty, sour, swimming pool water at this dose.
An alternative is to add 1 cup of apple juice or less to the Chlorine Dioxide rather than water. Apple juice contains only 2.2 mg of vitamin C per cup so it will deactivate only a tiny portion of the Chlorine Dioxide. Taking the Chlorine Dioxide with apple juice makes it much more palatable for young children or people who are very ill.
What to Expect When Taking Chlorine Dioxide
Dr. Hesselink does not attribute side effects to any direct effects caused by the oxidant medicine. Rather, transient discomforts caused by taking chlorine dioxide are due to the fact that the oxidants quickly kill large quantities of pathogens. The detoxification reaction that occurs is due to the death of pathogens that slowly disintegrate and release antigens that cause inflammation in the body.The severity of a detoxification reaction depends on the number of pathogens that are killed by the chlorine dioxide as well as the sensitivity of the immune system and the antigenicity of the waste that they leave behind.
Detox symptoms that are commonly reported when using chlorine dioxide include:
- Fever
- Headache
- Nausea
- Sweating
- Fatigue
- Diarrhea
- Chills
- General malaise
- Pain
- Body aches
Again, these effects are not side effects of taking Chlorine Dioxide. Rather, these symptoms are a sign of a detoxification reaction due to the fact that Chlorine Dioxide kills many pathogens quickly which can cause a bottleneck of detoxification the body must do quickly. If you experience these symptoms, lower your daily dose of Chlorine Dioxide by half. As soon as the symptoms disappear, start increasing your dose again slowly. The goal is to not cause a detoxification reaction, but to still administer therapeutic doses of Chlorine Dioxide to the body each day to produce a healing effect.
Troubleshooting Chlorine Dioxide: Foods Supplements and More That Can Inhibit Chlorine Dioxide
- You administered too little of the chlorine dioxide.
- The pathogen is resistant to chlorine dioxide.
- The pathogen is able to convert to alternative forms to avoid being killed by chlorine dioxide (e.g. cysts, eggs, spores, etc.)
- The pathogen is located in a remote place inside the body and the chlorine dioxide cannot reach it. In this case add dimethylsulfoxide (DMSO) to the solution (see the information on Dimethylsulfoxide / DMSO). NOTE: Add 3 times the number of drops of DMSO to your final dose of Chlorine Dioxide only after you have activated it for 3 minutes with the acid solution and then added water for optimal therapeutic results.
- Too many antioxidants were in the body at the time of treatment which cancelled out the effects of the chlorine dioxide.
Foods and Substances That Can Deactivate Chlorine Dioxide
There are a number of foods and substances that should not be present in the stomach at the moment when you administer chlorine dioxide. If you take an oxidant medication like Chlorine Dioxide by mouth with an antioxidant present in the stomach at the same time, these two classes of medicines will cancel each other out. Antioxidants of all kinds are the most important type of compound to avoid while taking Chlorine Dioxide. Below is a list of foods and substances to avoid while medicating yourself with Chlorine Dioxide:- N-Acetyl-Cysteine (NAC)
- All ascorbates
- Glutathione
- Alpha-Lipoic Acid
- Ascorbic Acid
- Anthocyanidins
- Polyphenols
- Benzaldehyde
- Tocopherols (including vitamin E)
- Quercetin
- BHT
- BHA
- Cinnamaldehyde
- Bioflavonoids
- Juice concentrates
- Many herbs including:
- Chocolate
- Silymarin
- Teas
- Coffee
- Gingko
- Licorice
- Olive
- Most fruits, particular berries, and grapes
- Fruit juices
- Fruit concentrates
- Wines
- Green drinks
- Brassica species
- Cabbage
- Kale
- Broccoli
- Cauliflower,
- Turnip,
- Mustard
- Wasabi
- Certain B vitamins
- Thiamine
- Riboflavin
- Pantothenate
- Folate
- Para-aminobenzoic acid
- Cyanocobalamin
- Biotin
- Carnosine
- Proteins (which quench the Chlorine Dioxide)
- L-tyrosine
- L-methionine
- L-cysteine
- L-tryptophan
- L-proline
- L-histidine
- Wheat germ
- Nuts
- Fish
- Poultry
- Meat
- Wheat germ
- Sulfur-rich foods
- Onion
- Garlic
- Shallot
- Peas
- Leeks
- Chive
- Egg
- Beans
- Milk
- Asparagus
- White potato (due to the presence of Alpha-Lipoic Acid)
Foods That Can Be Eaten During Chlorine Dioxide Treatment
It is best to eat foods that won’t react with chlorine dioxide during treatment to ensure that as many oxidants remain active as possible. The following foods are a good choice for anyone who is currently taking chlorine dioxide. These foods can be eaten at the same time as the chlorine dioxide treatment:- Nutrient poor white starchy foods
- White bread
- Grits
- Cassava
- White wheat pasta
- White rice
- Saltine crackers
Chlorine Dioxide and DMSO According to Dr. Hesselink
Dr. Hesselink talks in depth about how Chlorine Dioxide reacts with sulfur thiols, but he specifically notes that Chlorine Dioxide probably doesn’t react with oxidized sulfur compounds like Dimethylsulfoxide (DMSO), MSM, taurine, or sulfate. Indeed, adding three times the amount of DMSO to the activated Chlorine Dioxide mixture will help the medicine reach areas of the body that may otherwise be inaccessible.Final Thoughts
Dr. Hesselink broadly describes four categories of dosing for Chlorine Dioxide that patients may opt to follow:- Regular dosing (daily) of the INACTIVATED sodium chlorite solution (which becomes active in the body when it comes into contact with the hydrochloric acid in the stomach)
- Occasional (daily, monthly, weekly) dosing of INACTIVATED sodium chlorite
- Regular dose of ACTIVATED sodium chlorite (aka Chlorine Dioxide)
- Occasional (daily, weekly, monthly, etc.) dosing of ACTIVATED sodium chlorite (aka Chlorine Dioxide)
In all cases, Dr. Hesselink recommends that patients start with 0.25 mg of sodium chlorite per kg of body weight daily or less. This amount should be increased to a maximum of 2 mg daily as long as the patient does not have a strong detoxification reaction at this dose. Starting at the lowest dose is particularly important for patients who are very ill or weak. People with chronic, debilitating diseases may experience a better healing trajectory if they give themselves less frequent doses of Chlorine Dioxide to avoid a strong detoxification reaction. The organs are better able to heal when they can detoxify the body quickly.
Store the citric acid solution in a dark-colored bottle that is separate from the sodium chlorite solution. Both bottles should be stored a dark-colored bottle in a cool, dark place (a refrigerator would be ideal).
Note that Chlorine Dioxide is much stronger as a medicine than sodium chlorite solution that is unactivated. Sodium chlorite dosing may be especially warranted when the patient is not sick, but rather trying to prevent an illness such as cancer, influenza, or COVID-19. Using inactivated sodium chlorite solution rather than Chlorine Dioxide may be valuable as a way to prevent nausea and less severe detoxification reactions.
Regular dosing of Chlorine Dioxide may be disadvantageous in that patients are constantly exposed to oxidants and they are not as able to receive beneficial nutrients and antioxidants or drug therapies. Long-term use of Chlorine Dioxide could deplete the patient’s natural oxidant sensitive molecules and cause oxidative stress. Indeed, if you continuously dose yourself with a strong oxidant like Chlorine Dioxide every day, diseased cells will have a hard time healing themselves through the natural physiological process of antioxidant adaptation. Extremely low daily dosing, on the other hand that is not able to overcome antioxidant adaptation, on the other hand, may be too weak to kill pathogens.
Warning for Children, Infants, and Pregnant Women Who Use Chlorine Dioxide Chronically
Children, infants, or fetuses who are repeatedly exposed via public drinking water to Chlorine Dioxide levels above 0.8 mg/Liter in public drinking water may experience nervous system damage. In other words, children who are exposed to high water purification levels of Chlorine Dioxide at every meal and every time they drink water suffer adverse effects. On the other hand, as a parent, if you use Chlorine Dioxide as a medicine, your child will be exposed to Chlorine Dioxide for short periods of time. For example, if you give your child Chlorine Dioxide for 3 weeks to cure an infection and then you give your child 5 to 7 days off from the medicine, your child will likely be able to catch up on nervous system growth/healing during the time off from the medicine. One of the major differences between fully grown adults and children, pregnant women, and infants, is the fact that pregnant women, children, and infants are all engaged in rapid growth. Give your child time off from daily Chlorine Dioxide treatments to grow. Use other alternative curative medicines during the week that you take off from Chlorine Dioxide if your child has cancer or another serious disease. Most of all, use your intuition to decide what’s best for your body and your child’s body.Thyroid Hormones and Chlorine Dioxide Treatment
Thyroid hormones T3 and T4 are both phenols. As such, they are both susceptible to destruction by Chlorine Dioxide. In other words, Chlorine Dioxide can reduce the availability of thyroid hormones in the body. As such, it is usually not recommended that patients use Chlorine Dioxide daily for long periods of time except when they have a severe, life-threatening infection or disease such as HIV, cancer, diabetes, COVID-19 (only take Chlorine Dioxide for the first 2-3 days until coughing begins), dental issues, meningitis, etc. The seriousness of the disease may be greater than the risk of thyroid hormone destruction in these cases. Use your best judgment if you plan to use Chlorine Dioxide daily to treat / cure a chronic disease over a long period of a time.That being said, this author has used Chlorine Dioxide for many diseases and disorders from COVID-19 and malaria to cancer and influenza. I do not use it daily, but I also don’t hesitate to take Chlorine Dioxide if I’ve been exposed to an infectious disease. On the other hand, I have HIV-positive friends who have used Chlorine Dioxide long-term on a daily basis for years to get off their other, much more damaging pharmaceuticals. As with all medicines, the ideal situation is where you are able to regain your health such that you don’t have to take any medicine every day. In some situations, that isn’t possible. If you have a chronic disease that requires daily use of Chlorine Dioxide, moderate the dose and take the lowest dose possible to still achieve the medicinal effects that are necessary for you to experience the highest possible quality of life.
If you intend to take Chlorine Dioxide long term, sodium chlorite that has not had the acid activator added to it is less reactive inside the body because it is in an alkaline state. As such, the INACTIVATED sodium chlorite solution is less reactive than Chlorine Dioxide toward human cells. INACTIVATED sodium chlorite solution is therefore safer than Chlorine Dioxide as a long-term medicine for use in humans and animals.
Chronic Fatigue Syndrome: Chlorine Dioxide Treatment
Dr. Hesselink recommends using a weekly dosing protocol at first for those with Chronic Fatigue Syndrome. Chronic Fatigue Syndrome patients can increase their dose of Chlorine Dioxide very slowly over time, but the goal is for them to experience longer and longer remissions from the disease.Cancer: Chlorine Dioxide Treatment
Dr. Hesselink theories that Chlorine Dioxide is one of the best possible treatments for cancer. He suggests, however, that due to the necessity for repeated dosing, sodium chlorite solution may be better tolerated than activated Chlorine Dioxide. Indeed, if you follow a high pH (alkaline) therapy as a cancer cure in addition to taking Chlorine Dioxide, the Chlorine Dioxide will be able to selectively seek out cancer cells more easily and react to them because many cancer cells secrete high levels of lactic acid or carbonic acid. When the Chlorine Dioxide is consumed by a patient with a relatively high pH, the Chlorine Dioxide is not activated by healthy human cells but when it comes into contact with cancer cells, the acids they produce activate the solution and cause it to become reactive. In other words, cancer cells activate the sodium chlorite producing oxidants that are more potent.Healthy Human Cells That Naturally Produce Acids That Activate Chlorine Dioxide
Though it is possible to alkalize the body such that Chlorine Dioxide is more reactive with cancer cells, there are a number of healthy human cells that also produce acids even if the body has been alkalized via an alkaline therapy. Examples include the following:- Isometric contraction of muscles causes muscle cells to produce large quantities of lactic acid and carbonic acid.
- Human cells that have a reduced blood supply anywhere in the body either due to obstruction of arteries or due to inflammation may also have a build-up of acids.
- Kidneys may have a build-up of acids for excretion from the body.
- Parietal cells in the stomach produce hydrochloric acid that is necessary for the digestion of food.
A patient who takes a high dose of sodium chlorite should pay special attention to muscle tissues. Schedule isometric exercise during a time when the body is not being exposed to high doses of sodium chlorite.
If you have muscle or back pain, this is a sign that the blood supply may be lower in those areas of the body. In other words, pain is a sign of ischemia or lower blood supply and/or inflammation. For example, if you have chronic shoulder pain, this area of your body likely has a higher acidity level than other areas of the body. As such, sodium chlorite is likely to be more reactive in these areas and could cause further oxidative damage in high doses. High doses of sodium chlorite could also cause oxidative damage to kidneys or stomach tissues given that these areas of the body are often acidic.
Kidney damage could be minimized by taking alkalizing supplements that would reduce the problem of acidic urine. Indeed, it may be possible to protect the stomach by taking a teaspoon of baking soda with sodium chlorite when it is taken by mouth.


Resources:
 Click here to sign up for the CDS Protocols App!
Click here to sign up for the CDS Protocols App!